Yes, it’s great having STP calculators, but sometimes you have to figure things out for yourself.
Before we do some calculations, let’s first define the 4 systems for comparing gas volumes that are currently being used.
| # | Name | Temp K | Temp C | Pres Atm | Pres Torr |
|---|---|---|---|---|---|
| 1 | IUPAC Before 1982 STP | 273.15 | 0 | 1 | 760 |
| 2 | IUPAC After 1982 STP | 273.15 | 0 | .98692 | 750.06 |
| 3 | NIST Uses NTP (Normal Temp & Pres) | 293.15 | 20 | 1 | 760 |
| 4 | SATP (Standard Ambient Temperarure & Pressure) | 298.15 | 25 | .98692 | 750.06 |
1) The first IUPAC method listed (replaced in 1982) defined STP as 273.15 K and 760 Torr.
Even though this is an old system, this method is still being reported on many websites and a great many Internet calculators continue to use it.
Also, some websites continue to state the old value of molar volume as 22.4139 liters without mentioning the new value.
2) In 1982, the International Union of Pure and Applied Chemistry (IUPAC) changed the pressure criterion to 750.06 Torr but left the temeperature criterion unchanged.
With the new criteria for STP, the new value of molar volume is 22.7109 liters.
3) The National Institute of Standards and Technology (NIST) uses a standard called “Normal Temperature and Pressure” (NTP) (293.15K and 760 Torr).
With these criteria, the value of molar volume is 24.0458.
4) For Standard Ambient Temperature & Pressure (SATP) the criteria are 298.15K and 750.06 Torr.
Under these criteria, the molar volume value is 24.789.
STP Calculations
1) A gas has a volume of 13.2 liters under the conditions of the old definition of STP (273.15 K and a pressure of 760 torr).
What is its volume under the new STP definition?

Volume at STP = 13.2 * (273.15 / 273.15) * (760 / 750.06)
Volume at STP = 13.37493 liters
2) A gas has a volume of 5.9 liters at STP (new definition 273.15 K and a pressure of 750.06 torr).
What is its volume at Normal Temperature and Pressure?
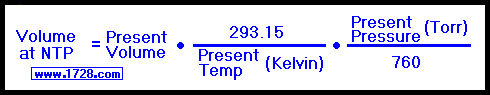
Volume at NTP = 5.9 * (293.15 / 273.15) * (750.06 / 760)
Volume at NTP = 6.2491812 liters
3) A gas has a volume of 7.9 liters at a temperature of 50° C and a pressure of 770 torr.
What is its volume at Standard Ambient Temperature and Pressure?

Volume at SATP = 7.9 * (298.15 / 323.15) * (770 / 750.06)
Volume at SATP = 7.482599 liters